Drug Interaction Risk Checker
Your body processes medications differently based on your genes, age, and other medications. This tool estimates your potential risk of adverse drug reactions using factors discussed in the article.
Have you ever taken the same medication as someone else and had a totally different experience? One person feels fine, another ends up in the hospital. It’s not just bad luck. It’s biology. Medications don’t work the same way for everyone-and the reasons why are more complex than most doctors even realize.
It’s Not Just About the Drug
When you take a pill, it’s easy to assume the drug itself is the only thing that matters. But the real story happens inside your body. Two people can take the same dose of the same medicine, and one gets relief while the other gets sick. Why? Because your body processes drugs differently than mine. And that difference isn’t random-it’s written into your genes.Up to 95% of how you respond to a drug comes down to your unique biology. That’s not a guess. It’s backed by decades of research. Some people break down drugs too fast. Others can’t break them down at all. And that changes everything-from how well the drug works to whether it causes dangerous side effects.
Your Genes Are the Hidden Switch
The biggest player in this game? Your liver enzymes. Specifically, the CYP450 family of enzymes. These are the body’s main drug processors. There are dozens of them, but three-CYP2D6, CYP2C9, and CYP2C19-are responsible for handling more than half of all commonly prescribed medications.Here’s the catch: up to 15% of adverse drug reactions are directly caused by genetic differences in these enzymes. For example, about 5 to 10% of white people are poor metabolizers of CYP2D6. That means their bodies can’t break down drugs like codeine, beta-blockers, or some antidepressants properly. The result? Toxic buildup. They get side effects even at normal doses.
On the flip side, 1 to 2% of Europeans-and nearly 30% of Ethiopians-are ultra-rapid metabolizers. Their bodies clear drugs so fast that the medicine never reaches effective levels. A person with this trait might take a standard dose of antidepressant and feel nothing. They’ll assume it’s not working and up the dose-risking overdose when they finally hit a normal metabolizer.
And it’s not just liver enzymes. Genes that control how drugs bind to their targets matter too. For example, warfarin, a blood thinner, needs a very precise dose. Too little, and you risk a clot. Too much, and you bleed internally. Genetic variants in CYP2C9 and VKORC1 explain 30 to 50% of why people need wildly different doses. In fact, patients who get genetically guided dosing cut their risk of dangerous bleeding by 31%.
Age, Illness, and Other Hidden Factors
Genes aren’t the whole story. Your body changes over time. As you get older, your fat percentage goes up. Your liver and kidneys slow down. That means drugs that stick to fat-like some antidepressants and sleeping pills-build up in your system. A 65-year-old might need half the dose of a 30-year-old, even if they’re the same weight.And if you’re sick? Inflammation from an infection or chronic condition can shut down your liver enzymes by up to 50%. That’s like putting a brick on your body’s drug-processing system. A drug that normally works fine might suddenly become toxic.
Then there’s polypharmacy-the habit of taking five, six, or more medications at once. That’s common in older adults. But each drug can interfere with another. Amiodarone, a heart medication, can block warfarin metabolism and boost its levels by 200 to 300%. That’s not a small risk. That’s a life-threatening one.
Real Cases, Real Consequences
This isn’t theoretical. It’s happening every day.A 68-year-old woman in a JAMA study kept having dangerous bleeding episodes on warfarin. Her INR kept spiking above 10-far above the safe range. Doctors kept adjusting her dose, but nothing helped. Finally, genetic testing revealed she was a CYP2C9*3/*3 poor metabolizer. Her dose was cut by 60%. Within weeks, her INR stabilized. She stopped going to the ER.
In asthma, a genetic variant in the 5-lipoxygenase gene affects about 5% of patients. For them, leukotriene modifiers like zafirlukast work wonders-improving lung function by 45%. But for the other 95%? The drug does almost nothing. And yet, it costs $250 to $300 a month. That’s tens of millions of dollars wasted every year on prescriptions that don’t help.
A 2022 Mayo Clinic study tracked 10,000 patients. Those who got genetic testing before starting certain drugs had 32% fewer ER visits and 26% shorter hospital stays. Simple. Effective. Life-changing.
Why Isn’t Everyone Getting Tested?
If the science is this clear, why aren’t we doing this routinely?The answer? It’s not about the science. It’s about the system.
The FDA has included pharmacogenomic info on over 300 drug labels. For 44 of them, they give specific dosing advice based on genetics. But only 18% of U.S. insurers cover the tests. Most hospitals don’t have the software to alert doctors when a patient’s genes conflict with a prescribed drug. And 68% of physicians say they don’t feel trained to use the results.
Even when testing is available, doctors don’t always act on it. Take clopidogrel, a common heart drug. About 2 to 15% of people are poor metabolizers of CYP2C19. For them, clopidogrel barely works-they’re still at high risk for heart attacks. But routine testing? Still not standard. Why? Because it’s not mandatory. And because it’s easier to just prescribe and hope.
The cost of testing has dropped from $2,000 in 2015 to around $250 today. That’s cheaper than a single specialist visit. Yet most people still get no genetic check before starting a new drug.

The Future Is Already Here
Change is coming. Fast.Medicare started covering pharmacogenomic testing for 17 high-risk drugs in January 2024. The FDA approved the first point-of-care CYP2C19 test that gives results in under an hour. In Europe, all new clinical trials must now include pharmacogenomic analysis. Oncology departments lead the way-65% use genetic testing routinely. Psychiatry and cardiology are catching up.
The next big leap? Polygenic risk scores. Instead of looking at one or two genes, these tools analyze hundreds at once. Early data shows they can predict drug response 40 to 60% better than single-gene tests. That’s huge. Especially for drugs like antidepressants or painkillers, where dozens of genes play a role.
At St. Jude Children’s Research Hospital, using pharmacogenomics to guide mercaptopurine dosing in leukemia patients cut severe side effects from 25% to 12%. That’s not just better outcomes. That’s fewer deaths.
What You Can Do Right Now
You don’t need to wait for your doctor to order a test. Here’s what you can do today:- If you’ve had a bad reaction to a drug-even a mild one-tell your doctor. Write it down. Include the name, dose, and what happened.
- Ask if your medication has known genetic interactions. Check the FDA’s list or ask your pharmacist.
- If you’re on five or more medications, ask about drug interactions. Ask if any of them affect liver enzymes like CYP2D6 or CYP2C19.
- Consider a pharmacogenomic test if you’re starting a long-term drug like warfarin, clopidogrel, or an antidepressant. The cost is low. The benefit can be life-saving.
This isn’t about being a medical expert. It’s about being an informed patient. You have a right to know why a drug might hurt you-and how to avoid it.
It’s Time to Stop Guessing
The era of ‘one-size-fits-all’ prescribing is over. We know now that your genes, your age, your other meds, and even your last cold all shape how a drug affects you. Ignoring that isn’t just outdated-it’s dangerous.Medicine is moving toward personalization. The tools are here. The data is clear. The only thing missing is the will to use them.
Don’t wait for your doctor to bring it up. Ask. Push. Protect yourself. Because your body doesn’t respond like anyone else’s. And that’s not a flaw. It’s biology. And it deserves to be respected.
Why do some people have side effects from a drug while others don’t?
It’s mostly due to genetic differences in how the body processes drugs. Key enzymes like CYP2D6 and CYP2C19 vary between people-some break down drugs too slowly (leading to buildup and toxicity), while others clear them too fast (making the drug ineffective). Age, other medications, inflammation, and even diet can also change how your body handles a drug.
Which drugs are most affected by genetic differences?
Warfarin (blood thinner), clopidogrel (antiplatelet), statins (cholesterol drugs), certain antidepressants like SSRIs, codeine (painkiller), and some asthma medications like leukotriene modifiers. The FDA has labeled over 300 drugs with pharmacogenomic information, and 44 include specific genetic dosing guidelines.
Is pharmacogenomic testing worth it?
For people on long-term, high-risk medications-like warfarin, clopidogrel, or antidepressants-it often is. Studies show it reduces emergency visits by 32% and hospital stays by 26%. The test costs around $250 now, down from $2,000 a decade ago. For someone who’s had bad reactions before, it’s one of the smartest health decisions they can make.
Can I get tested without a doctor’s order?
Some direct-to-consumer tests are available, but they’re not always clinically validated. For medical decisions, it’s best to get testing ordered by a doctor or pharmacist who can interpret the results in context. A genetic test without clinical guidance can lead to confusion or unnecessary worry.
Does insurance cover pharmacogenomic testing?
Coverage is improving but still limited. As of 2024, Medicare covers testing for 17 high-risk drugs. Private insurers vary-only about 18% offer full coverage. Tests are often covered if prescribed for a specific drug with known genetic interactions, like warfarin or clopidogrel. Always check with your insurer before testing.
What if I’m already on a drug and didn’t get tested?
It’s not too late. If you’re doing well and have no side effects, you may not need testing. But if you’ve had unexpected reactions, poor results, or are on multiple medications, ask your doctor about a pharmacogenomic test. It can help fine-tune your current treatment and prevent future problems.
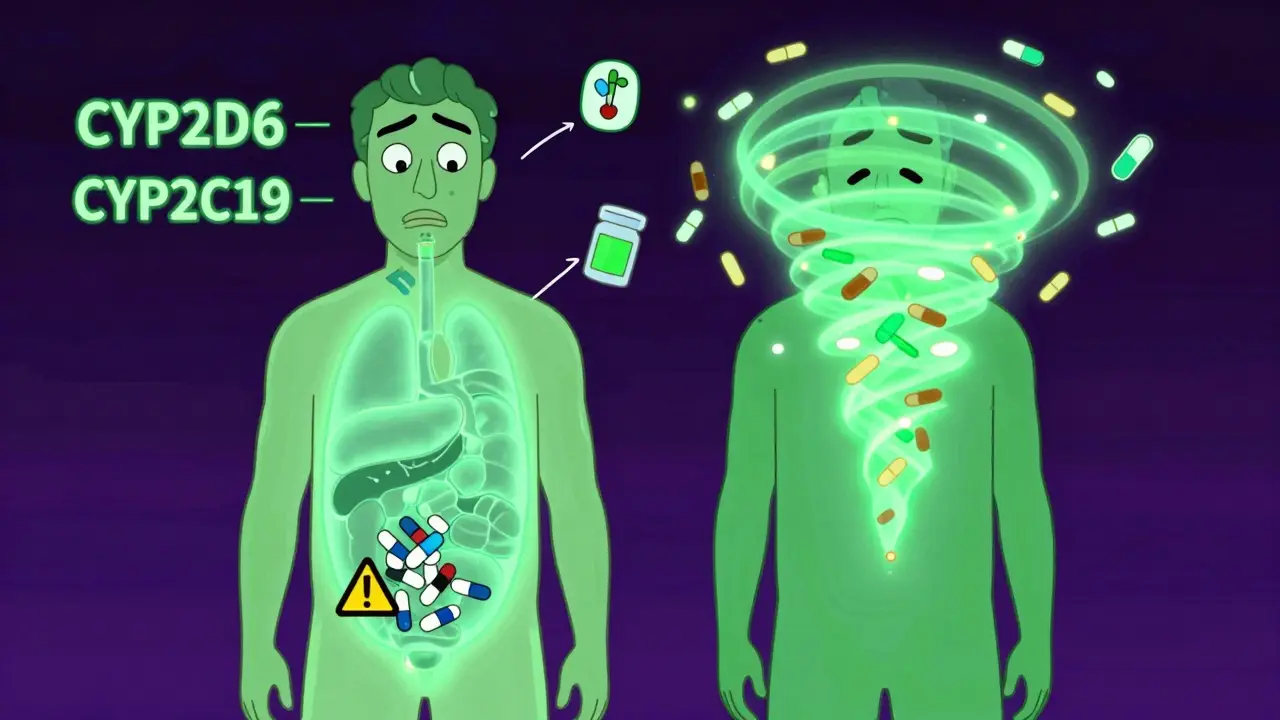
I took fluoxetine for years and kept feeling worse, not better. My doctor thought I was just 'not responding'-turns out I'm a CYP2D6 poor metabolizer. Once they switched me to something else, it was like a fog lifted. Genetic testing saved my sanity. If you're on antidepressants and it's not working, ask about this. It's not in your head-it's in your genes.
Let’s be brutally honest: this is a corporate shill disguised as science. The FDA ‘recommends’ genetic testing because pharmaceutical companies want to lock you into lifelong dependency on expensive, patent-protected drugs. Why not just fix the damn system instead of selling $250 tests to patch a broken model? And don’t get me started on how 68% of doctors are ‘untrained’-that’s not incompetence, that’s negligence enabled by a profit-driven healthcare machine.
This made me feel seen. I’ve been on warfarin for 8 years and had two scary INR spikes. No one ever mentioned genetics until I asked. Now I know I’m CYP2C9*3/*3. It’s scary to think how close I came to something worse. Thank you for writing this. I hope more people hear it.
While the scientific foundation of pharmacogenomics is robust and well-documented, the implementation challenges remain significant. The disconnect between clinical evidence and systemic adoption is not a failure of knowledge, but of infrastructure, reimbursement policy, and interdisciplinary coordination. Until electronic health records are interoperable with pharmacogenomic databases and clinical decision support tools are universally integrated, the potential of personalized prescribing will remain underutilized. This is a systems problem, not a patient problem.
Okay, but what about the 30% of people who are ultra-rapid metabolizers and think their antidepressant isn’t working so they double the dose… and then wake up in the ER? I know someone who did this. She thought she was being proactive. Turns out she nearly poisoned herself. And the doctor? Said, ‘Weird, that’s not supposed to happen.’
Why aren’t pharmacies flagging this? Why isn’t the script itself saying, ‘WARNING: CYP2D6 Ultra-Rapid Metabolizer Risk’? This isn’t just science-it’s a public safety issue. Someone’s gonna die because no one’s talking about this.
Oh, so now we’re supposed to believe that Big Pharma didn’t design this entire system to keep us dependent on their $500/month drugs? Genetic testing? Please. They’ll just charge you $2,000 for the test, then bill you for the ‘personalized’ drug that costs 3x more. And don’t get me started on how they’ll use your DNA data to sell you ads for ‘supplements that fix your CYP2C19’-oh wait, they already are. This isn’t medicine. It’s surveillance capitalism with a lab coat.
My grandma took 7 meds. One made her dizzy. Another gave her nausea. She thought it was just ‘getting old.’ Then we found out two of them blocked the same liver enzyme. She cut one, swapped another-now she’s hiking again. 🙌 Genes + meds = real life. Ask your doc. It’s worth it.
Oh wow, another ‘science’ article that ignores the fact that 90% of these ‘genetic differences’ are statistically insignificant when you control for diet, sleep, stress, and environmental toxins. You’re blaming genes because it’s easier than admitting your entire medical model is built on placebo-level evidence. And let’s not forget: 70% of these ‘life-saving’ genetic tests are never replicated in independent studies. Wake up.
I work in a rural clinic. We don’t have genetic testing. But we do have patients who tell us, ‘I can’t take that pill-it made me feel like I was going to die.’ We listen. We adjust. We trial alternatives. Sometimes, the most powerful tool isn’t a lab report-it’s a patient who knows their own body. That’s not anti-science. That’s human-centered care. And yes, genetic testing should be more available. But let’s not forget: we’ve been doing this right for decades, even without the tech.